Integrated metabolism
Learning outcome 1: To explain some basic concepts of metabolism
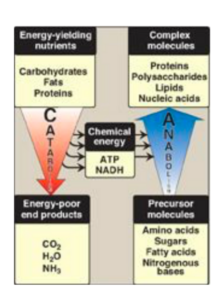
Introduction: Metabolism is an integrated network of biochemical reactions in the body that involves different biochemical pathways. These pathways can be classified as catabolic (degradative), breaking down complex molecules into simpler ones, or anabolic (synthetic), forming complex molecules from simple precursors. Catabolic reactions break down molecules such as proteins, carbohydrates, and lipids into simpler molecules such as CO2, NH3, and water, while anabolic reactions form end products from simple precursors.
Anabolic, catabolic and amphibolic pathways:
- Anabolic pathways are metabolic processes that involve the synthesis of complex molecules from simple precursors. They require energy input and are essential for growth, repair, and maintenance of the body’s tissues. Examples of anabolic pathways include protein synthesis, glycogen synthesis, and lipid synthesis.
- Catabolic pathways are metabolic processes that involve the breakdown of complex molecules into simpler components. They release energy and are important for generating ATP, the body’s primary source of energy. Examples of catabolic pathways include cellular respiration, glycolysis, and beta-oxidation of fatty acids.
- Amphibolic pathways are metabolic processes that involve both catabolic and anabolic reactions. These pathways serve to integrate the metabolic pathways in the body, balancing energy production and utilization with the synthesis and storage of energy-rich molecules. An example of an amphibolic pathway is the citric acid cycle, which is part of cellular respiration and involved in the breakdown of glucose and the synthesis of some amino acids.
Learning outcome 2: To explain how metabolic pathways are regulated.
The regulation of intermediates in metabolic pathways is governed by four mechanisms:
- Substrate Availability: The availability of substrates in the metabolic pathway affects its flow.
- Allosteric Activation and Inhibition: Enzymes can be activated or inhibited by the binding of small molecules to sites that are distinct from the active site. This affects rate-determining reactions, such as the fructose-2,6-bisphosphate activator of phosphofructose kinase in glycolysis.
- Covalent Modifications of Enzymes: The activity of enzymes can be altered by the addition or removal of phosphate groups. For example, glycogen phosphorylase, fructose bisphosphatase, and hormone-sensitive lipase are inactive in their dephosphorylated state.
- Induction-Repression of Enzyme Synthesis: The synthesis of enzymes can be increased (induced) or decreased (repressed), affecting the amounts of enzymes available and changing the flow of intermediates in metabolic pathways. For example, increased insulin levels lead to an increase in the synthesis of key enzymes involved in anabolic metabolism.
Learning outcome 3: To describe the alterations of metabolism in the well-fed state and in starvation.
Metabolism in a well-fed state:
Absorptive State: The 2- to 4-hour period after the ingestion of a normal meal is referred to as the absorptive state. During this time:
- Plasma glucose, amino acids, and triglycerides (TG) levels increase
- Insulin levels increase and glucagon levels decrease, leading to an increase in the synthesis of TG, glycogen, and proteins
- All tissues use glucose as a metabolic fuel
- The metabolic response of the body is primarily influenced by alterations in the metabolism of the liver, adipose tissue, muscle, and brain.
Early Fasting State:
- Blood-glucose level drops several hours after meal
- Insulin secretion decreases
- Glucagon secretion increases, leading to a decrease in blood-glucose level
Glucagon:
- Indicates a starved state
- Mobilizes glycogen stores
- Inhibits glycogen and fatty acid synthesis
- Stimulates gluconeogenesis in the liver
- Maintains blood-glucose level
Muscle and liver switch to using fatty acids as fuel when blood-glucose levels drop.
Metabolism during Fasting State:
Starvation can occur due to an inability to obtain food, the desire to lose weight rapidly, or clinical situations such as trauma, surgery, neoplasms, or burns. During fasting, plasma levels of glucose, amino acids, and triglycerides decrease. This decrease leads to a change in the insulin to glucagon ratio, which results in the degradation of triglycerides, glycogen, and protein.
This sets into motion an exchange of substrates among the liver, adipose tissue, muscle, and brain, guided by two main priorities:
- Maintaining adequate plasma levels of glucose to sustain energy metabolism in the brain and other glucose-requiring tissues.
- Mobilizing fatty acids from adipose tissues and ketone bodies from the liver to supply energy to other tissues.
During starvation, substrates are not provided by the diet but are instead available from the breakdown of tissues such as the degradation of triglycerides and the release of fatty acids from adipose tissue.
Fuel storage:
- Large amounts of energy can be stored as triglycerides (TG) in the body, compared to the small amounts stored as glycogen.
- Only a limited amount of the body’s protein (about one third) can be utilized for energy production without affecting critical body functions.
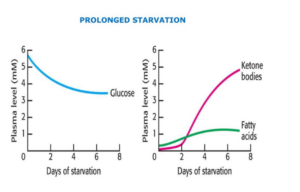
Prolonged starvation:
- After three days of starvation, the liver forms large amounts of ketone bodies and the brain and heart start to use them as fuel.
- After several weeks of starvation, ketone bodies become the major fuel for the brain.
- After depletion of TAG storage, protein degradation accelerates leading to the loss of heart, liver, and kidney function, resulting in death.
Learning outcome 4: To describe the metabolic inter-relationship between the major organs in the well-fed state and in starvation.
The liver: The nutrient distribution centre
After a meal, the liver takes in absorbed nutrients such as carbohydrates, lipids, and proteins. These nutrients are then processed, stored or routed to other tissues. The metabolism of the liver is affected by the elevated levels of insulin secreted by the pancreas.
A. Carbohydrate Metabolism:
- The liver retains 60g of every 100g of glucose delivered by the portal system.
- Glucose metabolism is increased by phosphorylation, increased activity of the hexose monophosphate pathway (HMP), and the upregulation of glycolysis and the TCA cycle.
- Gluconeogenesis is suppressed.
B. Lipid Metabolism:
- Fatty acid synthesis and TG synthesis are favored by the availability of substrates and the activation of acetyl CoA carboxylase.
- TG synthesis is enhanced by the presence of acetyl CoA, NADPH, and chylomicron remnants in the blood.
C. Protein Metabolism:
- Amino acid degradation increases, providing carbon skeletons for pyruvate, acetyl CoA or TCA cycle intermediates.
- Protein synthesis is upregulated to replace any proteins that may have been degraded in the previous postabsorptive state.
Adipose Tissue: Energy Storage Depot
- In a 70 kg man, adipose tissue weighs approximately 14 kg.
- In obese individuals, adipose tissue can constitute up to 70% of body weight.
- Adipocytes can be nearly entirely occupied by a droplet of TG.
Carbohydrate Metabolism:
- Glucose uptake is stimulated by increased insulin.
- Glycolysis supplies glycerol phosphate for TG synthesis.
- The activity of Hexose Monophosphate Pathway (HMP) is increased.
Lipid Metabolism:
- Synthesis of TG is increased with the availability of fatty acids from hydrolysis of TG in chylomicrons and Very Low Density Lipoprotein (VLDL).
- Degradation of TG is decreased with increased insulin favoring the dephosphorylated state of hormone-sensitive lipase.
Skeletal Muscle: A Key Consumer of Oxygen
- At rest, muscle accounts for about 30% of the body’s oxygen consumption
- During vigorous exercise, muscle can consume up to 90% of the total O2 consumed
Carbohydrate Metabolism
- Glucose uptake is increased by the presence of insulin
- Glycogen synthesis is enhanced by insulin and increased glucose-6-phosphate
Lipid Metabolism
- Fatty acids (FA) are released from chylomicrons and VLDL by lipoprotein lipase
- FA are of secondary importance as a fuel source for muscle during the well-fed state, where glucose is the primary source of energy
Protein Metabolism
- Protein synthesis replaces degraded proteins since the previous meal
- Branched-chain amino acids (leucine, isoleucine, valine) are taken up by muscle, used for protein synthesis and as energy sources.
The Brain: the control centre of the body
- Accounts for 2% of adult weight but 20% of basal O2 consumption
- Glucose is the primary fuel in the fed state
- During starvation, ketone bodies can serve as alternative fuel
Glucose Metabolism
- The brain uses glucose exclusively as fuel, oxidizing approximately 140 g/day to CO2 and H2O
- Does not have significant glycogen stores and is dependent on availability of blood glucose
- Substrates must be able to pass the blood-brain barrier to provide energy.